| OMIM: | 613670, 300672 |
| Diagnostic method: | Sequencing: ARHGEF9, ATRX, CUL4B, KDM5C, PHF6, PQBP1, SLC6A8; CNV analysis ATRX, SLC6A8 |
| Samples required: | 2 ml EDTA blood |
| Duration of analysis: | 6-8 weeks |
| Forms: |
Mental retardation describes the developmental disorder with intellectual disabilities. Affected patients are characterized by impaired cognitive and adaptive abilities and show a reduced intelligence (IQ <70). The prevalence in the population is about 2%. Severe forms with an IQ <50 occur much less frequently and are given with a prevalence of ~0.4%. The clinical picture of mental retardation is very heterogeneous and is still not completely understood. It is currently assumed that genetic factors play a role of at least 50% in the development of mental retardation. Additional psychological and somatic comorbid diseases or signs of dysmorphism often occur.
The diagnosis of patients with mental retardation takes place in a stepwise diagnostic approach. About 10-20% of affected patients have a chromosomal aberration. Therefore, after the clinical indication, a chromosome analysis is carried out at the beginning of the diagnosis, whereby aneuploidies e.g. Trisomy 21, Turner Syndrome or smaller chromosomal aberrations such as partial trisomies can be detected. Another 10-15% with a normal chromosome analysis have causal microdeletions or duplications. These chromosome aberrations are analyzed in a second diagnostic step using array CGH. The results to date have shown that such microdeletions or duplications are often detected in people with autism spectrum disorders (ASD) but also in patients with intellectual disabilities. Autistic disorders comprise a group of profound developmental disorders of the brain that are present from birth with the appearance of symptoms in the first few years of life. More recent studies determined a worldwide prevalence for autism spectrum disorders (ASD) of around 0.8% across all age groups. The men are significantly more often affected than women (4:1). Three common signs (core symptoms) can be observed in ASD patients: impaired social behaviour, impaired communication and language skills and stereotyped behaviors and interests. In addition to the appearance of the core symptoms, ASD patients show numerous psychological and somatic comorbid diseases or signs of dysmorphism.
Overall, in about 65% of patients with mental retardation the above methods are not able to detect a genetic cause for the nonsyndromic developmental disorders. Recent studies suggest that patients often have dominant new mutations (de novo) in genes that play an essential role in the development and networking of neurons. The risk of such de novo mutations increases with the paternal allele, whereas the rate of chromosomal trisomies increases with the maternal allele. Research in recent years suggests that up to 50% of severe developmental disorders are due to de novo mutations and smaller deletions / duplications (indels), with the distribution of genetic aberrations being very heterogeneous.
In our molecular genetic diagnostics, we first carry out an analysis of the fragile X syndrome, which is the most common monogenetically inherited disease in patients with mental retardation, followed by panel diagnostics with the basic autism panel or basic X-linked retardation panel according to the medical indication, if the result is inconspicuous,. Due to the genetic heterogeneity of the disease, the last step is a trio analysis using a clinical exome. Therefore, an extended analysis of 6794 disease-relevant genes is used. In order to identify dominant de novo, the parents are also analyzed in this diagnosis.
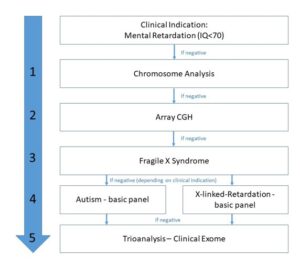
Diagnostic:
Step 1: Chromosome Analysis
Step 2: Array CGH
Step 3: Fragile-X-syndrome
Step 4: NGS-basic panel (<25kb): X-linked Mental Retardation or Autism Spectrum Disorder
Step 5: Clinical exome (Trio analysis)
